Pax Pharmaceutica
ASEAN Covid situational report. XBB.1.5 spreads rapidly in US. Paxlovid questions? Big Pharma is not re-investing profits. China is cautious on US XBB.1.5 and speeds medicine manufacture
UPDATE: If you don’t know already, the Covid-XBB.1.5 sub-variant is spreading like wildfire across the US. The Long Mekong Weekend Edition swallows the pill and provides a diagnosis of the Covid-19 situation in ASEAN and publishes the World Health Organisation’s (WHO) prognosis.
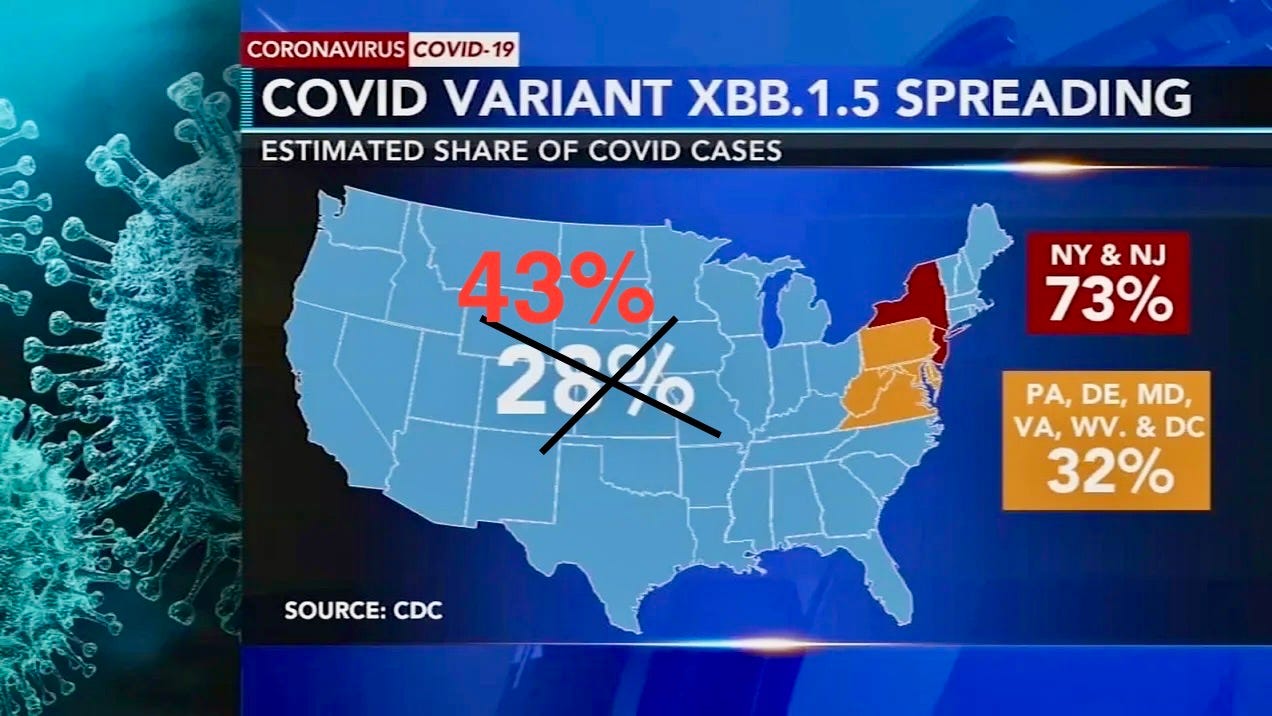
43% of all new US cases are XBB.1.5 and it has produced 82% of all global sequences.
Paxlovid is being promoted as the best medicine for infected patients, but whether or not it can speed recovery for omicron and its new sub variants, such as XBB.1.5, remains unclear. The US pharmaceutical industry - Big Pharma - is under scrutiny for excessive profit-taking, while obfuscating and obscuring its lower than reported investments in innovation and testing. In China, authorities are watching the US closely for signs of the new sub-variants arrival and taking all precautions to arrest the spread of XBB.1.5. Chinese pharmaceutical manufacturers have sought technical transfers to ramp up production and distribution of Covid medications for the expected rise in cases during the New Year holidays and celebrations.
The Long Mekong Weekend Edition is following WHO advice and wearing a mask in medium to high risk situations like planes and public transport, public spaces and social or business gatherings.
Worldwide, there have been over 656 million cases and over 6 million deaths attributed to COVID-19
In the USA the XBB.1.5 sub-variant is spreading rapidly reaching almost half of the 1.7 million new cases. Countries around the world are watching the Biden administration carefully as the US’ past performance on Covid spread does not bode well for it to take the necessary measures to control the spread of XBB.1.5 globally.
ASEAN has released a new report with country updates.
Download the full report here.
Regional Update
Cambodia's active COVID case total rose for the first time in 2023, as Prime Minister Hun Sen cautioned Cambodians of COVID variant XBB.1.5 - the most transmissible with the 'recombinant' new strain of Omicron called "Kraken".1 [Full Article]
Indonesia: Following the elimination of all remaining pandemic health restrictions at the end of last year, the government intends to devote its attention and resources to modernizing the country's healthcare system.2 At a news conference on January 5, Health Minister Budi Gunadi Sadikin stated that the country's daily caseload over the previous year had demonstrated that foreign varieties, which had previously caused considerable problems for Indonesia's pandemic handling, no longer posed a substantial threat.2 [Full Article]
Malaysia: To avoid the spread of COVID-19, Melaka residents are encouraged to wear face masks in public and crowded locations, according to Chief Minister Datuk Seri Sulaiman Md Ali. He advised individuals, including tourists, to take additional precautions such as physical separation, the use of hand sanitizers, and frequent hand washing. He stated that if the COVID-19 situation deteriorated, face masks might be made mandatory.
Philippines: Thousands of Catholic devotees, many wearing protective masks and carrying candles, marched through downtown Manila early of January 8 to honor a centuries-old black statue of Jesus Christ, which was not paraded to deter an even larger throng over COVID-19 worries.8 The nearly 6-kilometer (3.7-mile) "Walk of Faith" procession of more than 80,000 devotees, according to church officials, was a fraction of the more than a million worshippers who typically converged in pre-pandemic years to pay homage to the life-size Black Nazarene statue in one of Asia's largest religious festivals. The Philippines is one of Southeast Asia's hardest-hit countries by the epidemic. [Full Article]
Vietnam: Vietnamese Prime Minister Pham Minh Chinh has ordered the country's COVID- 19 prevention and control to be strengthened as the Lunar New Year approaches, with various festivals planned, local media reported on January 8.10 Though the pandemic has been mostly contained, it continues to evolve in a complex manner, with new variations such as XBB developing, he added in a statement.10 According to Xinhua, the Prime Minister directed the Ministry of Health to continue closely monitoring the pandemic situation and coordinating with appropriate agencies to respond proactively to pandemic developments. [Full Article]
WHO weighs in on Omicron XBB.1.5 subvariant; US extends COVID health emergency
The World Health Organization (WHO) advisory group on virus evolution said the Omicron XBB.1.5 subvariant is poised to drive an increase in COVID-19 cases, but it cautioned that confidence in its assessment is low, because most of the information is based on data from just one country—the United States.
In other developments, health officials in the United States extended the COVID-19 public health emergency again, as the world closely watches developments there with XBB.1.5 and looks for clarity on China's evolving surge.
In an update yesterday, the sequence sharing database GISAID said China continues to ramp up its genomic surveillance, with a host of genome sequences shared by provinces, cities, universities, and private labs. So far, the data suggest that the sequences from China resemble known circulating variants. New data from Shanghai show a range of known lineages from multiple separate introductions. GISAID also marked the third anniversary of its EpiCoV database, which now has 14.5 million sequences shared by 215 countries and territories.
More than 82% of XBB.1.5 sequences are from US
The WHO said 5,288 XBB.1.5 sequences have been reported between Oct 22 and Jan 11 from 38 countries. Just over 82% are from the United States, with the United Kingdom (8.1%) and Denmark (2.2%) among other countries reporting the most sequences. Last week, US officials fine-tuned their assessment of XBB.1.5 proportions, showing the spread is still robust.
Along with BQ.1 subvariants, XBB.1.5 is one of the most immune evasive variants to date, the WHO said. So far, there is no evidence that illnesses involving the subvariant are more severe, and XBB.1.5 doesn't carry mutations that are known to increase severity.
WHO has updated its guidelines on mask wearing in community settings, COVID-19 treatments, and clinical management. WHO continues to recommend the use of masks by the public in specific situations, and this update recommends their use irrespective of the local epidemiological situation, given the current spread of the COVID-19 globally. Masks are recommended following a recent exposure to COVID-19, when someone has or suspects they have COVID-19, when someone is at high-risk of severe COVID-19, and for anyone in a crowded, enclosed, or poorly ventilated space. Previously, WHO recommendations were based on the epidemiological situation.
Read the full report here.
Read full health guidelines here.
Paxlovid - nirmatrelvir / ritonavir
Paxlovid is a medicine used for treating COVID-19 in adults who do not require supplemental oxygen and who are at increased risk of the disease becoming severe. Paxlovid contains two active substances, PF-07321332 and ritonavir, in two different tablets.
Paxlovid can only be obtained with a prescription. The recommended dose is two tablets, each containing 150 mg PF-07321332, plus one tablet containing 100 mg ritonavir, to be taken together by mouth twice a day for 5 days. Paxlovid should be given as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of the start of symptoms.
A main study involving patients with COVID-19 and at least one underlying condition putting them at risk of severe COVID-19 looked at the effects of Paxlovid on rate of hospitalisation or death within 28 days of treatment when compared with placebo (a dummy treatment). The analysis was done in patients who received Paxlovid within 5 days after COVID-19 symptoms began and who did not receive nor were expected to receive treatment with antibodies. Over the month following treatment there were no deaths in the Paxlovid group and 12 deaths in the placebo group.
The majority of patients in the study were infected with the Delta variant. Based on laboratory studies, Paxlovid is also expected to be active against Omicron and other variants. The most common side effects with Paxlovid (which may affect less than 1 in 10 people) are dysgeusia (taste disturbance), diarrhoea, headache and vomiting.
Access the EU commission Paxlovid website here.
Paxlovid, the COVID antiviral developed by Pfizer, was hailed as a miracle drug against COVID-19 when it was approved for use by the FDA in December. But it was nowhere to be found during the Omicron wave that followed and now is little discussed and underused, with doses reportedly piling up on pharmacy shelves. Has Paxlovid failed to live up to the hype as a pandemic game changer, or is it another effective defense against COVID that’s been unjustly snubbed by a misinformed public?
“It’s probably wasted on the mildly ill,” he said. “Before we had Paxlovid, plenty of people who had mild symptoms would get over it and they were fine.”
For a frontline view I turned to my brother-in-law, John Emy, a doctor of internal medicine who practices with CareMount Medical in Manhattan and has been prescribing Paxlovid to his patients with COVID. He said he’s a fan — with qualifications. “I think it’s a great drug. It’s certainly very effective. It starts working pretty quickly,” he told me over the phone while walking to work. “Usually within 24 hours, the symptoms start to improve.” He wondered how badly it was really needed, though.
Read the full article here.
How Big Pharma Actually Spends Its Massive Profits
Pharmaceutical giants rang in the new year by quietly announcing price hikes in the United States on more than 350 drugs — and they continue to insist these price hikes are necessary for innovation. But new research shows that the business model of America’s largest pharmaceutical companies involves far more spending on enriching shareholders and executives than on research and development.
Between 2012 and 2021, the 14 largest publicly-traded pharmaceutical companies spent $747 billion on stock buybacks and dividends — substantially more than the $660 billion they spent on research and development, according to a new study by economists William Lazonick, professor emeritus of economics at University of Massachusetts, and Öner Tulum, a researcher at Brown University.
But that hasn’t stopped drug companies and their lobbying groups from using the cost of innovation as a key argument in their campaign to keep Medicare from being able to negotiate lower drug prices. The pharmaceutical industry has spent at least $645 million on federal lobbying over the past two years.
Read full article here.
Sick with “Shareholder Value”: US Pharma’s Financialised Business Model During the Pandemic
Evidence sharply contradicts PhRMA’s contention that its member companies need unregulated drug prices to generate profits that they then reinvest in drug innovation. On August 16, 2022, Congress passed the Inflation Reduction Act, which, among other things, enables Medicare to negotiate the prices of certain high-cost prescription drugs, beginning in 2026. Even though it is just one step forward in confronting US pharma’s financialised business model, this legislation was a long time coming.
A New York Times article published almost four decades ago reported accusations against pharmaceutical companies by then-US Rep. Henry Waxman (D-CA) of “outrageous price increases” and “greed on a massive scale.”
However, there is abundant—and indeed overwhelming—evidence that most of these pharmaceutical executives allocate corporate profits to massive distributions to shareholders in the form of cash dividends and stock buybacks. Rather than devoting the high profits from high drug prices to augmenting and accelerating investment in drug innovation, US pharmaceutical companies burden US patients and taxpayers with high drug prices so that, through massive distributions to shareholders, the senior executives who make these allocation decisions can boost the yields on the companies’ publicly traded shares.
Data for the 474 corporations included in the S&P 500 Index in January 2022 and publicly traded from 2012 through 2021 reveal that these corporations distributed $5.7 trillion as share repurchases during the 2012-2021 fiscal years, representing 55 percent of net income, and $4.2 trillion as dividends, an additional 41 percent of net income (see Table 1). The vast majority (we estimate about 95 percent of the total) of the share repurchases were done as open-market repurchases (OMRs) of common shares, the purpose of which is to manipulate the company’s stock price.
Read the full article here.
China monitors 1 XBB infection, faces continuous risk of imported cases
According to the monitoring data for coronavirus variants, China has found one XBB variant case domestically from December 1, 2022 to January 12, Chen Cao, a research fellow with the virology institute of the Chinese Center for Disease Control and Prevention (China's CDC), told a press conference on Friday.
China has monitored 33 imported XBB infections and its sub-branch from 20 countries and regions, showing the country is facing risks from imported XBB-related infections and sub-branch variants and related domestic infections. Currently, the XBB variant has not spread widely in China, according to Chen.
The main strains currently circulating in China are BA.5.2 and BF.7. The neutralizing antibodies produced by BA.5.2 or BF7-infected individuals remain at a high level in the short term, and offer better cross-protection against variants including XBB, China's CDC said in a previous press conference.
A new COVID variant known as XBB.1.5 is spreading fast in the US and threatening to cause further waves of infection. It's one of the latest descendants of Omicron, causing the number of cases in the US to more than double in a week at the end of December.
Read full story here.
China accelerates localized production of imported COVID treatments
Chinese authorities and manufacturers are actively promoting localized production of COVID-19 treatments as the country is making full preparations to protect high-risk groups, given potential epidemic waves during the upcoming Lunar New Year festival.
The US pharmaceutical giant Merck Sharp & Dohme (MSD)'s China branch announced on Wednesday that, to meet the needs of Chinese patients and help China's fight against COVID-19, MSD launched licensing negotiations with Sinopharm to manufacture and supply molnupiravir in China.
On Thursday, Sinopharm told media that molnupiravir would be available online in the Chinese mainland market since Friday, media reported. Sinopharm and MSD signed a cooperation framework agreement in September, under which Sinopharm would be a dealer and exclusive commission agent for MSD's antiviral COVID-19 medicine in China. The two sides would also negotiate the feasibility of a technical transfer under the framework so that the drug molnupiravir could be produced and provided in the Chinese mainland market. The two sides signed an agreement specifically on distribution later in November during the China International Import Expo in Shanghai. Liu Yong, president of Sinopharm, said at that time that the group would make full use of its distributing network and market service capacity to enhance the accessibility of the treatment in a short period.
Another major COVID-19 treatment producer Pfizer is also talking with a Chinese partner to produce and provide its COVID-19 treatment Paxlovid locally, the company's chief executive Albert Bourla told media during the JP Morgan Healthcare Conference. China-produced pills are expected to be available within the next few months. Pfizer's Chinese partner is reportedly the Zhejiang-based Chinese drugmaker Huahai Pharmaceutical, which announced in August 2022 that it had signed a deal with Pfizer to produce the drug in the Chinese mainland solely for patients in the country.
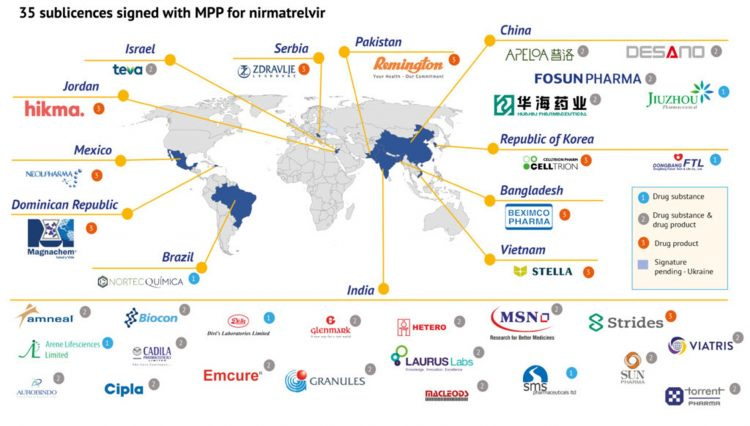
Huahai Pharmaceutical told media on Tuesday that it was actively working with Pfizer to accelerate localised production of Paxlovid. Huahai is one of the five pharmaceutical companies in China that have signed on with the UN-backed public health organization Medicines Patent Pool to manufacture the generic version of Paxlovid for supply in 95 low- and middle-income countries, the organization announced on Thursday. China was not included in the 95 countries. The pills Huahai would produce for the mainland market would not be the generic version, but the original version. The Huahai-produced Paxlovid would be available in the mainland market as early as the first half of 2023.
Read full story here.